HIV-AIDS – Immunity, Eradication and Its Disappearing Victims
Human immunodeficiency virus (HIV), the retrovirus responsible for acquired immune deficiency syndrome (AIDS) has been around since between 1884 and 1924 (while lentiviruses, the genus to which HIV belongs, have existed for over 14 million years) when it entered the human population from a chimpanzee in southeastern Cameroon during a period of rapid urbanization. At the time, no one noticed nor knew that it would result in one of the deadliest pandemics. Nor was anyone aware that some would possess a natural immunity, a cure would remain elusive a decade into the 21st century, and a significant number of deceased victims would be purged from mortality statistics distorting the pandemic’s severity.
As the number of cases spread from Cameroon to neighboring countries, namely the Democratic Republic of Congo (DRC), Gabon, Equatorial Guinea, and the Central African Republic, they drew little attention even as victims died in scattered numbers from a series of complications (e.g. Pneumocystis pneumonia (PCP), Kaposi’s sarcoma, etc.) later attributed to AIDS. This was likely because of Africa’s limited interaction with the developed world until the widespread use of air travel, the isolated, low incidence of cases, HIV’s long incubation period (up to 10 years) before the onset of AIDS, and the absence of technology, reliable testing methods and knowledge surrounding the virus. The earliest confirmed case based on ZR59, a blood sample taken from a patient in Kinshasha, DRC dates back to 1959.
The outbreak of AIDS finally gained attention on June 5, 1981 after the U.S. Centers for Disease Control (CDC) detected a cluster of deaths from PCP in Los Angeles and New York City. By August 1982, as the incidence of cases spread, the CDC referred to the outbreak as AIDS. The responsible retrovirus, HIV, was isolated nearly a year later (May 1983) by researchers from the Pasteur Institute in France and given its official name in May 1986 by the International Committee on Taxonomy of Viruses. During this period, HIV-related mortality rates rose steadily in the United States peaking in 1994-1995.
HIV:
HIV is spherical in shape and approximately 120 nanometers (nm) in diameter (or 60 times smaller than a red blood cell). It is composed of two copies of single-stranded convoluted RNA surrounded by a conical capsid and lipid membrane that prevents antibodies from binding to it. HIV also consists of glycoprotein (gp120 and gp41) spikes and is a highly mutating virus. Its genome changes by as much as 1% each year, significantly faster than “killer” cytotoxic T-Cells (CD8+) can adapt. It is transmitted through bodily fluids.
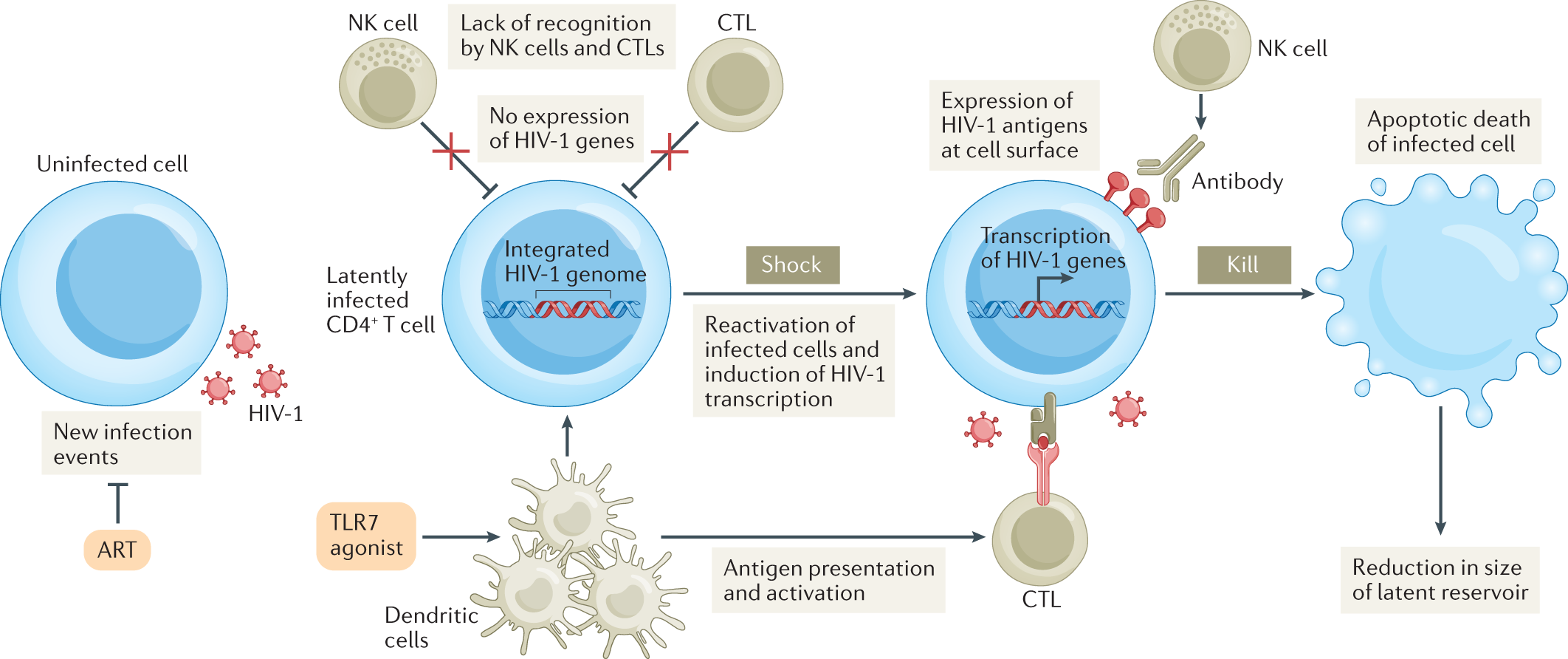
Per CD4 Cell Tests (Fact Sheet Number 124, AIDS InfoNet, 21 March 2009), when “HIV infects humans” it infects “helper” T-4 (CD4) cells that are critical in resisting infections. HIV does so by merging its genetic code with that of T-4 (CD4) cells. HIV’s spikes stick to the surface of T-4 (CD4) cells enabling its viral envelope to fuse with their membrane. Once fused, HIV pastes its contents into the DNA of T-4 (CD4) cells with the enzyme, integrase, so that each time T-4 (CD4) cells replicate, they produce additional “copies of HIV,” reducing the count of healthy T-4 (CD4) cells. Then as healthy T-4 (CD4) cells, which come in millions of families geared towards specific pathogens are eliminated, the body is rendered defenseless against the pathogens “they were designed” to fight until ultimately, the immune system is overwhelmed.
When the T-4 (CD4) cell count drops below 200 cells per cubic mm of blood (or a percentage of? 14% of total lymphocytes; normal counts range from 500-1600 or 30%-60% of lymphocytes), indicative of serious immune system damage, the victim is deemed to have AIDS (“the end point of an infection that is continuous, progressive and pathogenic per Richard Hunt, MD (Human Immunodeficiency Virus And AIDS Statistics, Virology – Chapter 7, Microbiology and Immunology On-line (University of South Carolina School of Medicine, 23 February 2010)) and is vulnerable to a multitude of opportunistic infections. Examples are PCP, a fungal infection that is a major killer of HIV-positive persons, Kaposi’s sarcoma, a rare form of cancer, toxoplasmosis, a parasitic infection that attacks the brain and other parts of the body and cryptococcosis, a fungal infection that attacks the brain and spinal cord (both usually occur when the T-4 (CD4) cell count drops below 100), and mycobacterium avium complex (MAC), a bacterial infection that can be localized to a specific organ (usually the bone marrow, intestines, liver, or lungs) or widespread, in which case it is referred to as disseminated mycobacterium avium complex (DMAC) (which often occurs when the T-4 (CD4) cell count drops below 50).
Natural Immunity:
Since the onset of the HIV/AIDS pandemic in 1981 cases of people with a natural immunity to HIV have been documented. Although these persons, called long-term non-progressors (LTNPs) are infected with HIV, they never develop AIDS. When LTNPs are infected, some suffer an initial drop in their T-4 (CD4) cell count. However, when their T-4 (CD4) cell count reaches around 500 it stabilizes and never drops again preventing the onset of AIDS. Furthermore, while CD8+ T-Cells (even in large numbers) are ineffective against HIV-infected T-4 (CD4) cells in progressors (persons without a natural immunity to HIV), the National Institutes of Health (NIH) reported in a December 4, 2008 press release that “CD8+ T-Cells taken from LTNPs [can efficiently] kill HIV-infected cells in less than [an] hour” in which “a protein, perforin (produced only in negligible amounts in progressors), manufactured by their CD8+ T-Cells punches holes in the infected cells” enabling a second protein, “granzyme B” to penetrate and kill them.
Per Genetic HIV Resistance Deciphered (Med-Tech, 7 January 2005) the roots of this immunity dates back a thousand years due to “a pair of mutated genes – one in each chromosome – that prevent their immune cells from developing [Chemokine (C-C motif) receptor 5 (CCR5) receptors] that let [HIV penetrate].” This mutation likely evolved to provide added protection against smallpox according to Alison Galvani, professor of epidemiology at Yale University. Based on the latest scientific evidence, the mutated CCR5 gene (also called delta 32 because of the absence or deletion of 32 amino acids from its cytokine receptor) located in Th2 cells, developed in Scandinavia and progressed southward to central Asia as the Vikings expanded their influence. Consequently up to 1% of Northern Europeans (with Swedes being in the majority) followed by a similar percentage of Central Asians have this mutation, which if inherited from both parents provides them total immunity while another 10-15% of Northern Europeans and Central Asians having inherited the mutation from one parent exhibit greater resistance in lieu of complete immunity to HIV.
At the same time, even though the CCR5 mutation is absent in Africans, a small also exhibit percentage natural immunity (possibly developed through exposure) to HIV/AIDS – CD8+ T-Cell generation that effectively kills HIV-infected cells and mutated human leukocyte group A (HLA) antigens that coat the surface of their T-4 (CD4) cells to prevent HIV from penetrating based on an intensive study of 25 Nairobi prostitutes who per The Amazing Cases of People with Natural Immunity against HIV (Softpedia, 27 June 2007) have “had sex with hundreds, perhaps thousands of HIV-positive clients” and shown no sign of contracting HIV.
In addition, people with larger numbers of the CCL3L1 gene that produces cytokines (proteins that “gum” up CCR5 receptors) to prevent HIV from entering their T-4 (CD4) cells, per Genetic HIV Resistance Deciphered have greater resistance to HIV in comparison to others within their ethnic group that possess lesser quantities of the CCL3L1 gene and get “sick as much as 2.6 times faster.”
At the same time, up to 75% of newborn babies also possess natural immunity (for reasons still not known) when exposed to HIV-positive blood. Although born with HIV antibodies – thus HIV-positive, newborns “usually lose HIV antibodies acquired from their HIV-positive mothers within 12-16 – maximum 18 months,” in which their “spontaneous loss of [HIV] antibodies” without medical intervention is called seroreversion. “However, with the exception of very few instances, these infants are not HIV-infected” conclusive proof of a natural immunity to HIV.[1] Furthermore, when pregnant HIV-positive women are administered highly active antiretroviral therapy (HAART), which lowers the viral concentration of HIV in their blood, an astonishing 97% of their newborns lose their HIV antibodies through seroreversion to become HIV-free per the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) as posted under Surveillance Monitoring for ART Toxicities Study in HIV-Uninfected Children Born to HIV-Infected Mothers (SMARTT) (Clinical Trials.gov, 29 March 2008). However, at this time, it is not known if these newborns retain their natural immunity throughout their lives.
Eradication:
With a cure perhaps unattainable, eradication of HIV/AIDS in the same way as smallpox (with no cure) was eliminated, may be the most feasible option. According to Dr. Brian Williams of the South African Centre for Epidemiological Modelling and Analysis, eradication of HIV/AIDS is an achievable goal that could be attained by 2050 if the current HIV/AIDS research paradigm is changed from focus on finding a cure to stopping transmission.
Per Dr. Williams such an effort would require testing billions of people annually. Though costly, the benefits would exceed the costs “from day one” according to the South African epidemiologist. Anyone found with HIV antibodies would immediately be administered antiretroviral therapy (which reduces HIV concentration 10,000-fold and infectiousness 25-fold) to halt transmission, effectively ending such transmission by 2015 and eliminating the disease by 2050 as most carriers die out, according to his estimate. The reason for this optimism, per Steve Connor, Aids: is the end in sight? (The Independent, 22 February 2010), is a “study published in 2008 [that] showed it is theoretically possible to cut new HIV cases by 95%, from a prevalence of 20 per 1,000 to 1 per 1,000, within 10 years of implementing a programme [sic] of universal testing and prescription of [HA]ART drugs.”
Even though clinical trials to test Dr. Williams’ vision will start in 2010 in Somkhele, South Africa, access to HAART still needs to be improved greatly to purge the disease. Presently only about 42% of HIV-positive people have access to HAART.
Furthermore, for eradication efforts to succeed, prevention programs (which currently reach fewer than 1 in 5 in sub-Saharan Africa, the epicenter of the pandemic where the average life-expectancy has fallen below 40 leaving about 15 million children orphaned) will have to continue to play an essential role in stopping transmission. Such programs though not limited to, must include abstinence, condom distribution, education re: transmission, safe sex, etc., and needle distribution to drug users (the latter which is badly lacking according to Kate Kelland, Failure to aid drug users drives HIV spread: study (Reuters, 1 March 2010) with “more than 90% of the world’s 16 million injecting drug users offered no help to avoid contracting AIDS” despite the fact that such users often share needles and approximately 18.75% are believed to be HIV-positive).
Proof that such efforts can work is evident when the President’s Emergency Plan for AIDS Relief (PEPFAR) created in 2003 for Africa that provides funding focused on HAART and palliative care for HIV/AIDS patients, HIV/AIDS awareness education and prevention programs (condoms, needle-exchanges, and abstinence) and financial assistance to care for the pandemic’s orphans and other vulnerable children, is considered. Per Michael Smith, PEPFAR Cut AIDS Death Rate in African Nations (Med Page Today, 6 April 2009), the program “averted about 1.1 million deaths [from 2004-2007]… a 10% reduction compared to neighboring African countries.”
The “Disappearing” Victims:
Despite reason for optimism based on Dr. Williams’ vision of eradication, the “disappearance” of HIV/AIDS victims is highly disturbing. In fact, when current statistics are compared to past statistics, more than 19 million victims or triple the number of murdered Holocaust victims (1933-1945) have been purged from the official record (effectively minimizing the severity of the pandemic) without as much as a whimper of protest, possibly because demographically speaking, a statistically-significant number of the deceased fall into groups that have been and continue to be the subjects of racial, gender, cultural, and even religious discrimination. In the words of Charles King, an activist who spoke in San Francisco on World AIDS Day in 2007, it is likely because HIV/AIDS has mainly “taken the lives of people deemed expendable”[2] the same mentality used to justify Hitler’s “Final Solution” and other pogroms.
Back on January 25, 2002 in AIDS Death Toll ‘Likely’ to Surpass That of Bubonic Plague, Expert Says in British Medical Journal Special Issue on HIV/AIDS (Kaiser Network), it was written, “AIDS – which has already killed 25 million people worldwide – will overtake the bubonic plague as the ‘world’s worst pandemic’ if the 40 million people currently infected with HIV do not get access to life-prolonging drugs…”
A year earlier, UNAIDS listed the global death toll as 21.8 million with an increase of 3.2 million in 2002. By 2003, based on statistics reported by the World Health Organization (WHO), UNAIDS, and U.S. Census Bureau as tabulated in The Global HIV/AIDS Epidemic: Current & Future Challenges by Jennifer Kates, M.A., M.P.A., Director HIV Policy, Kaiser Family Foundation the global death toll had risen to 28 million by February 2003. Add annual mortality statistics of 3 million (2003), 3.1 million (2004 and 2005), 2.9 million (2006), 2.1 million (2007), and 2 million (2008, the most recent complete year of reporting) per UNAIDS, and an estimated, conservative total of 1.4 million (if another 28% decline as occurred between 2006 and 2007 took place between 2008 and 2009) the global death toll for year-end 2009 would be roughly 45.6 million. Yet, when UNAIDS released its latest report in November 2009 as reported in the Mail & Guardian (South Africa, 24 November 2009) the worldwide death toll through 2008 was listed as “passing 25 million,” approximately 19.2 million below the actual mark.
Per AIDS cases drop due to revised data (MSNBC, 19 November 2007), the “disappearing” victims can be attributed to “a new methodology.” While this may make sense with regard to prevalence since “[p]revious AIDS numbers were largely based on the numbers of infected pregnant women at clinics, as well as projecting the AIDS rates of certain high-risk groups like drug users to the entire population at risk” versus the new methodology that incorporates data from “national household surveys,” it does not with regard to mortality figures which are calculated primarily from national AIDS registries and/or death certificates based on the presence of HIV, T-4 (CD4) cell counts below 200, and death caused by opportunistic AIDS-related infections resulting from such low T-4 (CD4) cell counts.
In retrospect, when viewing the approximate 45.6 million figure, few pandemics have killed more than HIV/AIDS – Smallpox (which had come in waves since 430 BC until the World Health Organization (WHO) certified its eradication in 1979), killed 300-500 million, Black Death/Bubonic Plague killed approximately 75 million from 1340-1771, and Spanish Influenza killed between 40-50 million from 1918-1919.
Optimism for the Future:
Until HIV/AIDS can be certified as eradicated by the WHO, despite the terrible economic toll it has taken, especially on sub-Saharan Africa (due to lost skills, shrinking workforces, rising medical costs) and other developing regions and its devastating toll in human lives and on families, there is reason for optimism.
As of December 2008, per UNAIDS, 33.4 million people are infected with HIV, a 1.2% increase from a year earlier with much of the rise attributed to a declining mortality rate due to a 10-fold increase in availability of HAART since 2004. About 2.7 million persons were newly infected in 2008, 18% and 30% decreases in new HIV infections globally since 2001 and 1996, respectively. In another promising sign, new HIV infections in sub-Saharan Africa, responsible for about 70% of all HIV/AIDS-related deaths in 2008, has fallen by 15% since 2001. At the same time, there were approximately 2 million HIV/AIDS-related deaths in 2008, a 35% reduction from 2004 levels when the global mortality rate peaked.
Presently, the HIV/AIDS pandemic has begun to decline or stabilize in most parts of the world. Declines have been recorded in sub-Saharan Africa and Asia (although the mortality rate is increasing in East Asia) while the pandemic has stabilized in the Caribbean, Latin America, North America and Western and Central Europe. The only part of the world where the HIV/AIDS pandemic is worsening is the Eastern European (especially in Ukraine and Russia) and Central Asian region.
The declines should continue as new methods of prevention and treatment are developed. Based on studies of NLTPs, a new class of treatments focused on genetic therapy to delete the necessary 32 amino acids from CCR5 receptors, elicit perforin and granzyme B production, and develop protease inhibitors to provide immunity to HIV and halt its spread may be developed in the future.
Though still a long way off and potentially very expensive (up to $20,000 per treatment), Drugs.com Med News reported in Gene Therapy Shows Promise Against HIV (19 February 2010) that when researchers removed immune cells from eight HIV-infected persons, modified their genetic code and reinserted them, the “levels of HIV fell below the expected levels in seven of the eight patients [with] signs of the virus disappear[ing] altogether in one” even though HAART treatment was halted. A study by UCLA AIDS Institute researchers, which removed CCR5 receptors by “transplanting a small RNA molecule known as short hairpin RNA (shRNA), which induced RNA interference into human stem cells to inhibit the expression of CCR5 in human immune cells” mimicking those of LTNPs through the use of “a humanized mouse model,” as reported on February 26, 2010 in Medical News Today in Gene-Based Stem Cell Therapy Specifically Removes Cell Receptor That Attracts HIV, showed similar success in that it resulted in a “stable, long-term reduction of CCR5.”
At the same time, as announced in HIV/AIDS drug puzzle cracked (Kate Kelland, Reuters, 1 February 2010), British and U.S. scientists succeeded (after 40,000 unsuccessful attempts) in growing a crystal to decipher the structure of integrase, an enzyme found in HIV and other retroviruses. This will lead to a better understanding how integrase-inhibitor drugs work and perhaps to a more effective generation of treatments that could impede HIV from pasting a copy of its genetic code in the DNA of victims’ T-4(CD4) cells.
Likewise, per Structure of HIV coat may help develop new drugs (Health News, 13 November 2009) scientists from the University of Pittsburgh School of Medicine “unraveled the complex structure” of the capsid coat (viewing its “overall shape and atomic details”) “surrounding HIV” that could enable “scientists to design therapeutic compounds” to block infection.
At the same time, researchers at the University of Texas Medical School may have finally discovered HIV’s vulnerability, per Achilles Heel of HIV Uncovered (Ani, July 2008) – “a tiny stretch of amino acids numbered 421-433 on gp120” that must remain constant to attach to T-4 (CD4) cells. To conceal its weakness and evade an effective immune response, HIV tricks the body into attacking its mutating regions, which change so rapidly, ineffective antibodies are produced until the immune system is overwhelmed. Based on this finding, the researchers have created an abzyme (an antibody with catalytic or helpful enzymatic activity) derived from blood samples taken from HIV-negative people with lupus (a chronic autoimmune disease that can attack any part of the body – skin, joints, and/or organs) and HIV-positive LTNPs, which has proven potent in neutralizing HIV in lab tests, thus offering promise of developing an effective vaccine or microbicide (gel to protect against sexual transmission). Although human clinical trials are to follow, it might not be until 2015 or 2020 before abzymatic treatments are available.
Elsewhere, International AIDS Vaccine Initiative (IAVI) scientists recently isolated two antibodies from a NLTP HIV-positive African patient – PG9 and PG16 (called broadly neutralizing antibodies (BNAbs) that bind to HIV’s viral spike composed of gp120 and gp41 to block the virus from infecting T-4 (CD4) cells. Per Monica Hoyos Flight, A new starting point for HIV vaccine design (Nature Reviews, MacMillan Publishers Limited, November 2009) “PG9 and PG16, when tested against a larger panel of viruses [HIV] neutralized 127 and 116 viruses, respectively” providing additional hopes for developing an effective vaccine and novel treatment regimens that induce the body to produce BNAbs, which currently only the immune system of NLTPs can create.
At the same time, studies of newborn seroreversion and medically induced production of human leukocyte group A (HLA) antigens that coat the surface of T-4 (CD4) cells could also eventually lead to anti-HIV vaccine that could protect billions of people.
In the meantime until such developments bear fruit, HAART (despite its mild side effects such as nausea and headaches in some and serious to life-threatening side effects in others) has proven to be highly effective in containing HIV with, per Gerald Pierone Jr., MD in The End of HIV Drug Development as We Know It? (The Body Pro: The HIV Resource for Health Professionals, 18 February 2010) reporting, “about 80% of patients [receiving HAART] reach an undetectable viral load.” Furthermore, greater access to antiretrovirals, per Drop in HIV infections and deaths (BBC News, 24 November 2009) “has helped cut the death toll from HIV by more than 10%” from 2004-2008 and saved more than 3 million lives based on UNAIDS and WHO statistics. HAART has also cut the age-adjusted mortality rate by more than 70% according to Kaiser Family Foundation’s July 2007 HIV/AIDS Policy Fact Sheet, because of its effectiveness in delaying and even preventing the onset of AIDS.
Despite HAART’s cost ($10,000-$15,000 per patient per year), the State of California in a report titled, HIV/AIDS in California, 1981-2008 called it “dramatic and life-saving” especially since early intervention results in greater mean T-4 (CD4) cell counts translating into fewer opportunistic infections and deaths. It also results in real cost savings because of the strong inverse relationship between T-4 (CD4) cell counts and associated medical expenses.
In conclusion, despite HIV/AIDS’ “disappearing” victims, there is reason for optimism. Research over the last year has offered several promising leads – the underlying cause of NLTPs’ immunity has been discovered, the structure of the HIV virus solved, and its weak point found – while improved access to HAART and HIV/AIDS education and prevention measures (with the exception of addressing intravenous drug users) have made significant inroads in reducing infection and mortality rates buying victims additional years and an enhanced quality of life.
______
[1] Orapun Metadilogkul, Vichai Jirathitikal, and Aldar S. Bourinbalar. Serodeconversion of HIV Antibody-Positive AIDS Patients Following Treatment with V-1 Immunitor. Journal of Biomedicine and Biotechnology. 7 September 2008.
[2] Michael Crawford. AIDS: Where is Our Rage? The Bilerico Project. 2 December 2007. 28 February 2010. http://www.bilerico.com/2007/12/aids_where_is_our_rage.php
Additional Source:
Wikipedia. 24-28 February 2010. http://en.wikipedia.org/